Introduction
Immunohistochemistry (IHC) detects and visualizes antigens in tissue sections using antibodies. It preserves the spatial context of proteins within tissues and enables researchers to study cellular localization, tissue architecture, and disease pathology in detail. This makes IHC a valuable tool in cancer research, neuroscience, and pathology.
Poor fixation, high background noise, or weak staining can compromise data integrity and reproducibility. Using high-quality reagents, such as those from Boston BioProducts, in combination with an optimized IHC protocol helps ensure strong, specific staining with minimal background interference.
Read on for a clear IHC step-by-step guide, troubleshooting tips, and best practices to help you confidently achieve precise, high-quality results.

Materials & Reagents
Here's everything you need to carry out our IHC protocol. In addition to Boston BioProducts' high-quality buffers and reagents, you'll need the following materials and equipment:
- Glass slides
- Coverslips
- Cryotome cryostat
- Microwave
- Antibodies (primary/secondary)
- Forceps
- Pipette and pipette tips
- Microscope
Boston BioProducts' Protocol
This IHC protocol walks you through tissue preparation, antigen retrieval, and visualization-even for paraffin sections and multiplexing-ensuring specificity, sensitivity, and reproducibility at every step.
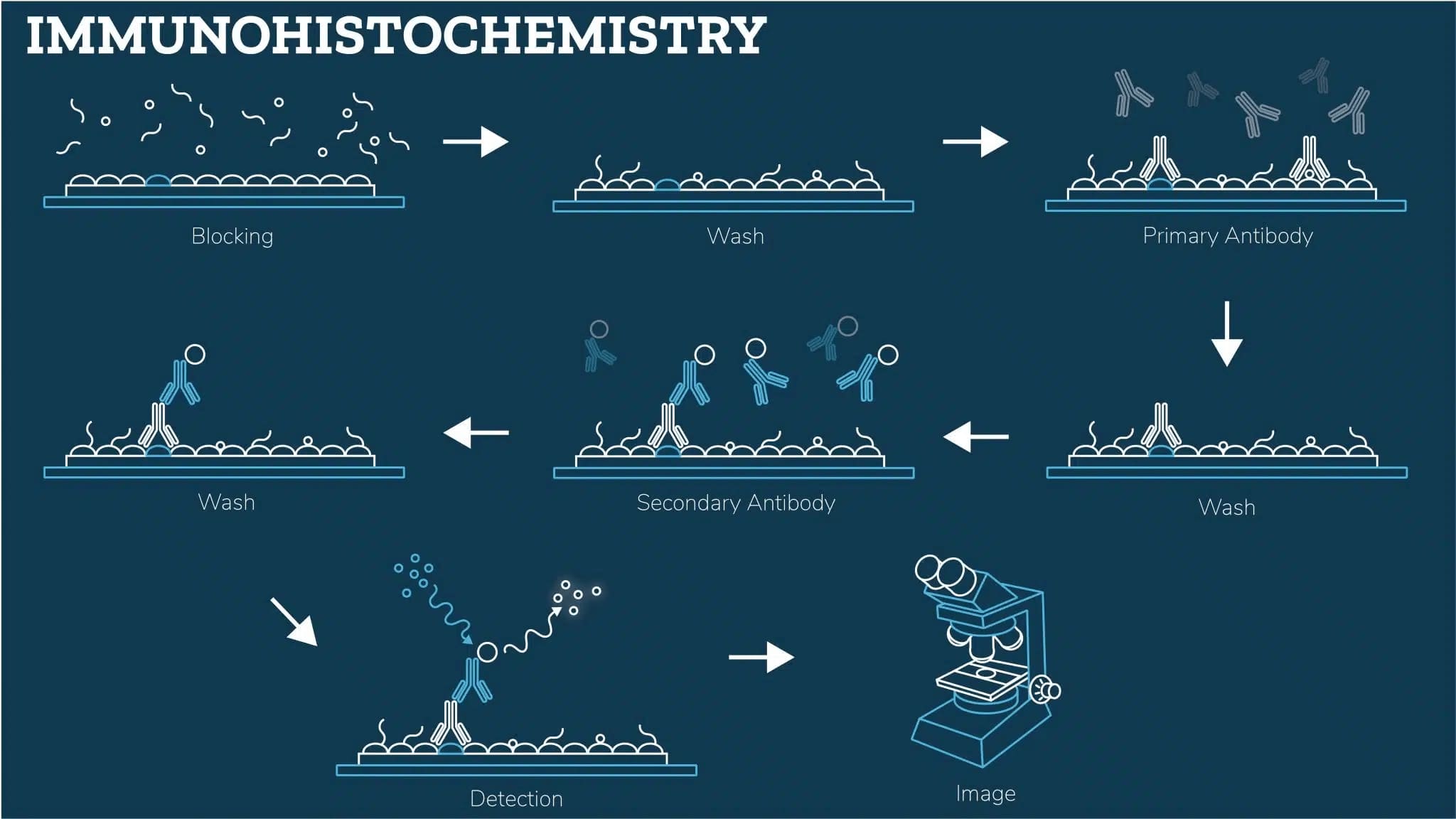
Tissue Preparation
Proper tissue preparation is crucial for successful IHC, as it preserves antigen integrity, maintains tissue morphology, and ensures consistent staining. In this section, we outline the essential steps for preparing fresh, frozen, or paraffin-embedded tissue samples.
For fresh or frozen tissue
Although IHC can be performed with fresh samples, most IHC protocols will be optimized for frozen or paraffin-embedded sections.
Transfer your frozen or fresh sample block to the cryotome cryostat, if using frozen tissue, allow the temperature to equilibrate first, and cut the tissue section to the desired thickness (typically 5-10 μm).
Keep your cryostat temperature between -15 and -23 °C to prevent curling or sticking.
Air dry (10-15 min) while preparing fixative.
Immerse samples in 3.7% paraformaldehyde (50-100x tissue volume) for 4-24 hrs at RT.
Wash the sections in PBS to remove the fixative.
Fixation temperature and time may require optimization depending on the tissue type and size.
Mini IHC protocol for paraffin sections
If you are working with paraffin-embedded tissue samples, you must take additional steps to prepare the tissue. This mini IHC protocol for paraffin sections outlines some key actions.
Tissue preparation
Deparaffinize in xylene (2x10 min, fresh solution each time), then rehydrate through ethanol (100% → 50%) and distilled water (2 min each).
Antigen Retrieval
Antigen retrieval is usually not required for fresh or frozen samples. However, for paraffin-embedded tissue, it is required to unmask antigens. The most common techniques are heat-induced epitope retrieval (HIER) and enzyme-induced epitope retrieval (EIER).
EIER is useful for antigens that may be damaged by high temperatures.
HIER: Heat slides in Citrate buffer or Tris-EDTA buffer (microwave, 10 min, medium power). Cool in buffer.
EIER: Incubate in Proteinase K Solution (10-20 min), then wash in PBS.
Monitor the buffer level during heating to prevent boiling over or evaporation, which may dry the slides out.
Prolonged Proteinase K incubation can damage tissue morphology, adjust time as necessary.
Permeabilization (optional)
After antigen retrieval, permeabilization is required if you are studying intracellular targets, as it helps antibodies penetrate cell membranes and access cytoplasmic and nuclear proteins.
Incubate your tissue sections in Triton X-100 or Tween-20 in PBS for 5-10 minutes at room temperature.
Triton X-100 is stronger and suited for nuclear targets, while Tween-20 is milder, so is better suited for cytoplasmic staining.
Over permeabilization can damage tissue morphology and lead to non-specific antibody binding.
Blocking
Effective blocking is essential to prevent non-specific binding, minimize background staining, and reduce false positive signals.
Over-blocking can reduce signal intensity. If you experience weak staining, try shortening blocking time.
Wash slides in TBST.
Repeat.
The blocking solution should not contain serum of the host animal of the primary antibody as this could result in a high background.
Antibody Incubation
After blocking, you can now stain tissue samples by direct detection (with an enzyme or fluorophore-conjugated primary antibody) or indirect detection (with both primary and secondary antibodies).
Chemogenic IHC (cIHC) uses an enzyme-conjugated secondary antibody (e.g., HRP or AP) that catalyzes a colorimetric reaction, whereas fluorescent or immunofluorescence IHC (IF-IHC) uses fluorophore-conjugated antibodies that emit fluorescence when excited by a specific wavelength of light.
Direct
Incubate your tissue sections in the diluted primary antibody overnight at 4°C.
To prevent the slides from drying out during incubation, cover them with a humidified chamber.
Indirect
Incubate your tissue sections in the diluted primary antibody overnight at 4°C.
Incubate at room temperature for at least 30 minutes.
Detection
Detection in IHC reveals the presence and localization of the target antigen. Depending on the labeling method, this can be achieved through chromogenic (, color-based) or fluorescent (IF-IHC, light-emitting) detection. Selecting the appropriate detection method depends on sensitivity requirements, signal stability, and imaging preferences.
Prepare your substrate solution for cIHC, or secondary antibody solution for IF-IHC.
If you are doing IF-IHC, make sure your tissue sections are protected from light throughout the process to avoid photobleaching.
Incubate your slides for as long as necessary.
For chromogenic detection, you can monitor the staining visually and finish when you reach the desired intensity.
Wash slides in PBS to remove excess substrate solution or unbound antibody.
Always include a negative control to detect any non-specific secondary antibody binding.
Mounting and Visualization
Proper mounting preserves the stained tissue for imaging and, if necessary, long-term storage. Your mounting protocol may differ depending on your tissue sample and type of IHC.
Paraffin-embedded sections require dehydration and a resin-based medium.
cIHC
Submerge your slides in hematoxylin (30 secs-2 mins).
Wash the slides with cool running water to blue the stain.
Dehydrate (ethanol 70% → 100%), clear with xylene.
Mount (5 min), coverslip, and image in brightfield microscope.
Use the smallest required volume of mounting media and make sure there are no air bubbles, as these can affect image quality.
IF-IHC
Counterstain (DAPI, 5 min), wash in PBS.
Mount with anti-fade medium, coverslip.
IF-IHC needs an anti-fade mounting medium, while cIHC uses resin-based mounting for long-term storage.
Image using the appropriate fluorescence filter for your fluorophore.
If you are storing your slides, you can seal the edges of the coverslip with clear nail polish to prevent drying and movement.
Looking for a multiplex IHC protocol?
Multiplex IHC detects multiple antigens in one section. Key differences are sequential antigen retrieval, adjusted blocking, fluorophore selection, and stripping buffer use.
Tissue preparation
Follow the tissue preparation steps in the protocol above for your relevant tissue type (fresh, frozen, or paraffin-embedded).
Antigen retrieval
Following tissue preparation, multiplex IHC may require you to perform antigen retrieval multiple times to prevent epitope masking or signal loss between rounds of staining.
Repeat the antigen retrieval steps (as detailed in the above IHC protocol) as many times as necessary.
Blocking
Apply blocking steps tailored to each antibody to minimize cross-reactivity and background staining.
Depending on your detection system, you may need to block endogenous peroxidases, phosphatases, and Fc receptors.
Sequential or simultaneous antibody staining
- Sequential staining: Apply the first primary antibody, followed by its detection system. Repeat this for subsequent antibodies.
- Simultaneous staining: If fluorophore-conjugated primary antibodies are used, ensure spectral separation to prevent bleed-through.
Signal amplification (optional)
For low-abundance targets, apply tyramide signal amplification (TSA) to enhance signal intensity while maintaining specificity.
Mounting and Visualization
Follow the main IHC protocol to mount your sections for imaging and storage according to the relevant method.
Troubleshooting & Optimization Tips for IHC Protocols

Get the most out of your IHC protocol by avoiding common issues like weak or inconsistent staining, high background noise, and non-specific binding, which compromise result quality.
My background staining is too high
The problem:
High background can be caused by non-specific antibody binding, inadequate blocking, or residual fixative in the tissue.
What to do:
My staining is weak or missing
The problem:
Weak or absent staining may be due to insufficient antigen retrieval, poor antibody binding, or low antigen expression.
What to do:
- Optimize your IHC deparaffinization protocol: If you are following an IHC protocol for paraffin sections, ensure that deparaffinization and rehydration steps are properly completed.
- Increase antibody concentration: Ensure the primary antibody is at the optimal concentration, and try extending the incubation time to enhance binding.
- Check tissue fixation: Over-fixation with formalin can mask antigens so, if necessary, reduce fixation time or try an alternative retrieval method.
- Try signal amplification: If you are following a multiplex IHC protocol, make sure you do the signal amplification step using TSA.
I have high non-specific staining
The problem:
Non-specific staining can arise due to antibody cross-reactivity, bleed-through in IF-IHC, or inefficient stripping between rounds of staining in multiplex IHC protocols.
What to do:
- Optimize antibody selection: To prevent cross-reactivity, ensure that the primary antibodies you’ve chosen are from a different host species.
- Adjust fluorophore selection: Ensure proper spectral separation to minimize channel overlap when imaging multiplexed stains.
- Use an effective stripping buffer: If sequential staining is performed in a multiplex IHC protocol, use a dedicated stripping buffer between rounds to remove previously bound antibodies.

Why Boston BioProducts?
Reagent quality is critical for achieving clear, specific staining and reproducible IHC results. Optimized antigen retrieval buffers ensure proper epitope exposure, while high-purity blocking solutions minimize background staining without interfering with target detection. pH-balanced wash buffers maintain tissue integrity and antibody binding, reducing variability between experiments.
By integrating Boston BioProducts' high-quality IHC buffers into your workflow, you can enhance signal clarity, improve specificity, and streamline your staining process-minimizing troubleshooting and ensuring consistent, high-quality results across every slide.

FAQs


