High-resolution separation of complex protein mixtures
Sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) is frequently used to obtain high-resolution separation of complex protein mixtures. However, poor band separation can occur due to issues at multiple steps, such as improper sample preparation or insufficient buffering capacity during gel electrophoresis. Complete lysis, improving protein solubility, and quality buffers can help deliver the clearly-resolved, straight bands you need for analysis and downstream applications.
Consult with Boston BioProducts to get high-resolution separation on your gels.
The Process
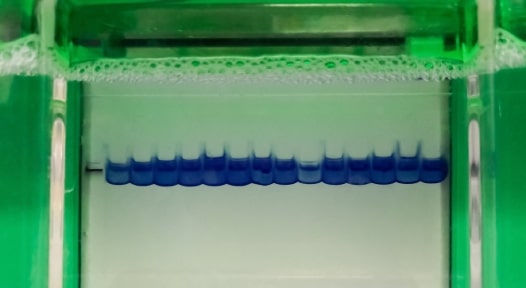
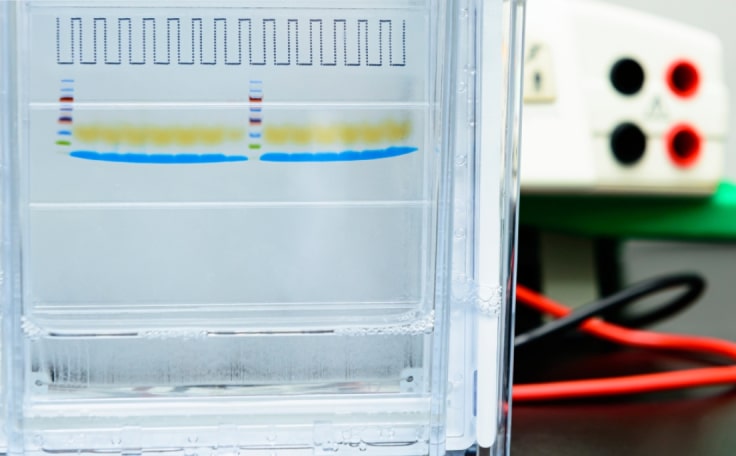
Lysed tissues or cells are denatured in SDS buffer containing loading dye. Once gels are cast, prepared samples are loaded onto the gel and then separated by size via polacrylamide gel electrophoresis (PAGE). Following resolution, proteins can be visualized by staining with Coomassie, silver, or fluorescent stains.
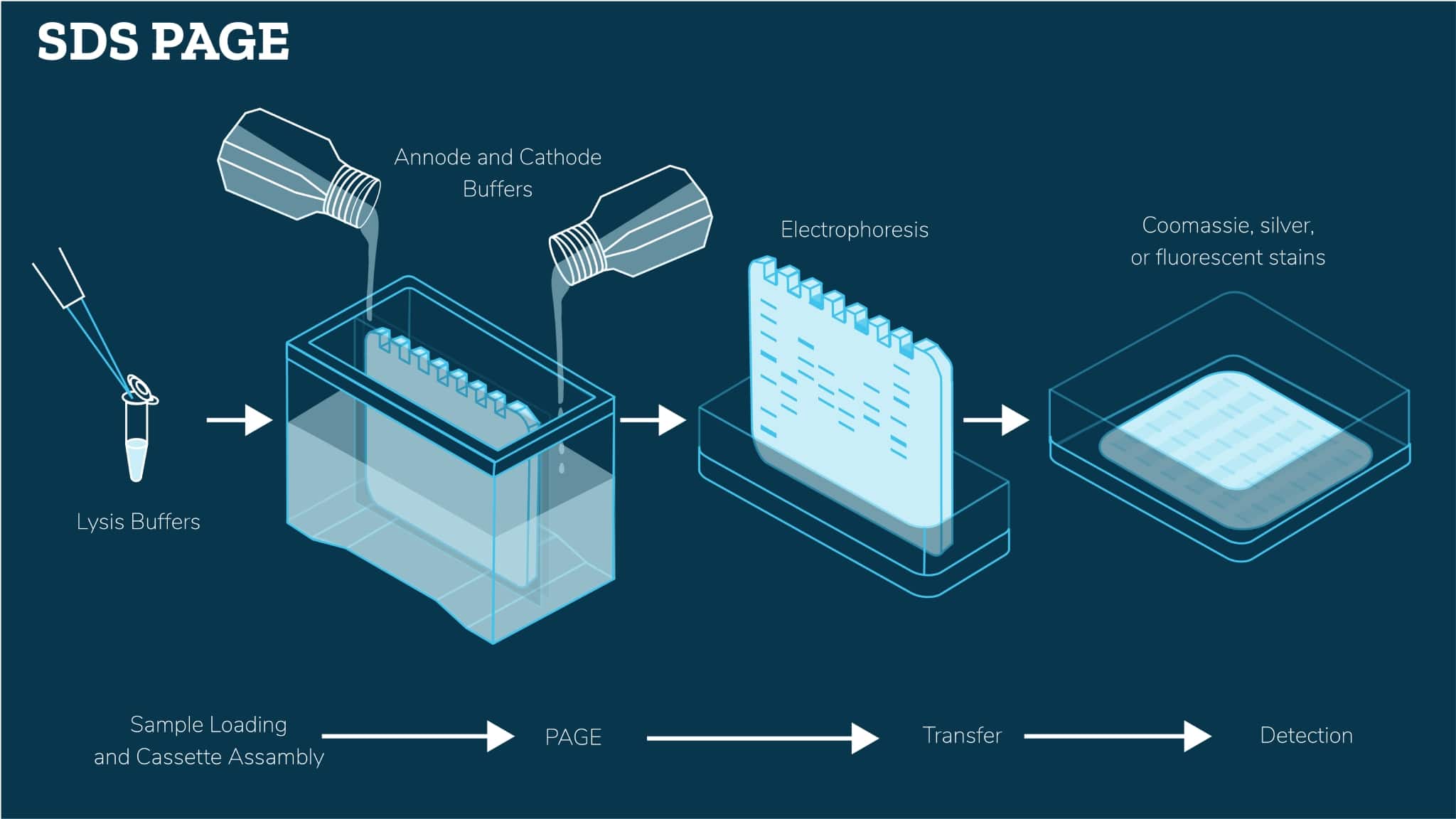
How we simplify the process
Most often when our customers contact us regarding difficulty in obtaining high-resolution separation of complex mixtures of proteins via SDS-PAGE, we look at optimizing the following parameters:
Lysis Buffer
It is essential that the samples (e.g., cells, tissues) are efficiently lysed. Identifying the right lysis buffer with the appropriate detergents for your protein(s) of interest is important. For example, membrane proteins require more thorough lysis to extract them from membranes.
SDS Sample Buffer
SDS in the sample buffer denatures proteins and gives them a negative charge to facilitate protein resolution during gel electrophoresis. Incomplete denaturation may lead to blurry bands, while insufficient negative charge will alter protein mobility.
Gel Casting Buffer
The pH of gel casting buffers will significantly influence SDS-PAGE protein resolution. Tris-Glycine gels are basic (pH 8.6) and over lower resolution compared to acidic Bis-Tris gels (pH 6.4). Tris-acetate gels, with a pH of 7, are best for very large proteins. The selection of running buffer depends on gel composition and the proteins of interest.
Staining Solutions
Visualizing resolved proteins, especially at low abundance, requires a sensitive staining method. Fluorescent stains will generally be the most sensitive.
Ways Boston BioProducts can help
Learn more about how
Boston BioProducts can help
Featured Products for SDS PAGE
Featured Lysis Buffers
Featured Gel Casting Buffers and Solutions
Featured Sample Buffers
Featured Electrophoresis Buffers
Featured Services & Resources

Quality Assurance
We vet reliable distributors, have a proven quality management system, and in-house manufacturing to ensure consistent high-quality products.

Certificate Request
Transparency is central to our approach to customer service and we want to ensure that you can trust your reagents.

Order Support
With a simple, reliable, and flexible ordering process, we prioritize getting your SDS PAGE reagents to you quickly and securely.
Frequently Asked Questions
The stacking gel has a lower concentration of acrylamide than the resolving gel, so proteins move through it more quickly. At the interface of the stacking and resolving gel, a pH gradient helps to "stack" the sample into a single band as it encounters the resolving gel. This stacking process improves band separation and resolution. The resolving gel has a higher concentration of acrylamide and a lower pH, causing proteins to move through it more slowly and separate proteins based on molecular weight.
Whether casting your own gels or purchasing precast gels is more suitable will depend on your needs. Some benefits of casting your own gels might include the ability to customize the gel composition, size, and thickness to meet your experimental needs, reduced cost, and flexibility to test multiple parameters or to change parameters frequently.
The concentration of acrylamide can be modified based on the protein(s) of interest. Larger proteins move more slowly through the gel than those with smaller molecular weights. Generally, low molecular weight proteins resolve best on higher percentage gels, while proteins with larger molecular weights should be run on lower gel concentrations. Gels can also be cast with a gradient of concentrations, which is particularly useful when there are multiple proteins of interest and they vary dramatically in size.
Gels can also be cast using different buffers (i.e., tris-glycine, tris-acetate, and bis-tris). Each of these is suitable for different proteins. Tris-acetate gels are the best choice for very large proteins. Bis-tris gels are the most stable and offer better resolution than tris-glycine gels.
When selecting a running buffer, depending on which gel recipe used, there are specific buffer requirements and not all standard buffers will work for all gels. Bis-Tris gels require either MOPS or MES buffer as a reducing agent must be present in the running buffer. These two buffers offer different banding patterns. Gels run with MES buffer run more quickly because MES has a lower pKa than MOPS and may be more suitable for smaller proteins. Alternatively, because MOPS runs more slowly, it may offer better resolution. Tris-glycine gels are generally run with tris-glycine buffer. Tris-acetate gels require tris-acetate buffer and will not work with MOPS or MES buffers.
Several stains can be used to visualize proteins on a gel. Coomassie, silver stain, and fluorescent stains are commonly used for this purpose. Coomassie staining can be done more quickly, while fluorescent staining and silver stain are more sensitive.